Abstract
Introduction: Anti-CD20 monoclonal antibodies (e.g. rituximab) have improved outcomes for patients with B-cell malignancies. Patients with follicular lymphoma (FL) have an excellent expected survival and frequently require multiple course of anti-CD20-based therapy, which has been associated with hypogammaglobulinemia (HG) and recurrent infections.
Objective: To evaluate the prevalence of HG and associated recurrent infections in patients with FL treated with anti-CD20-based therapy, and to describe the natural history of immunoglobulin (Ig) levels in groups of patients with different treatment intensities.
Patients and methods: This is a retrospective study of FL patients at Memorial Sloan Kettering Cancer (MSKCC) conducted with IRB approval. Patients with FL were identified in the MSKCC FL Database. The analysis was restricted to patients with ≥2 Ig assessments prior to and after anti-CD20-based therapy (largely rituximab). HG was defined as ≥1 documented IgG <600, IgA < 50 or IgM <50 mg/dL. Symptomatic IgG-HG was defined as ≥2 non-neutropenic infections (NNI) or ≥1 serious NNI (resulting in hospitalization, antibiotics, or initiation of intravenous Ig [IVIG]). For subsequent analyses, patients were classified into 5 groups based on exposure to anti-CD20 antibody. The control group consisted of patients with no exposure to anti-CD20 antibody, and included patients on active surveillance or treated with local IFRT. Group 1 received a single course of anti-CD20-based therapy (monotherapy [MONO] or chemoimmunotherapy [CI]). Group 2 received first line MONO or CI followed by maintenance with anti-CD20 antibody. Group 3 received multiple courses of MONO or CI. Group 4 included patients who received high dose therapy with autologous stem cell rescue (HDT/ASCR) and/or allogeneic stem cell transplant.
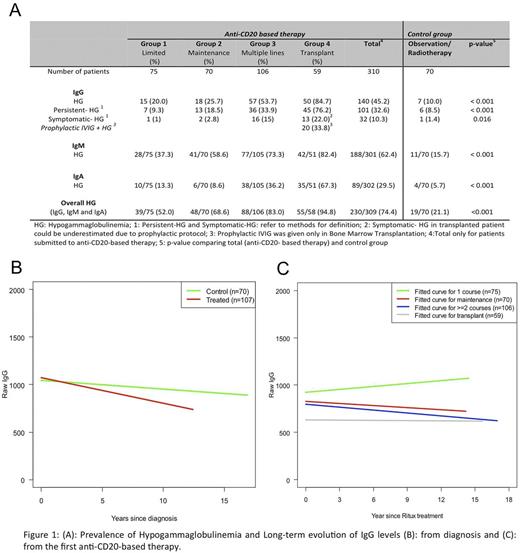
Results: Of 1123 consecutive patients at MSKCC diagnosed between 1998 to 2009 with FL, 380 were eligible for the analysis. Baseline characteristics were similar between eligible patients and the full dataset. 70 patients in the control group and 310 patients for the anti-CD20-based therapy group (75 patients in Group 1; 70 patients in Group 2; 106 patients in Group 3; 59 patients in Group 4). Exposure to anti-CD20-based therapy was associated with an increased risk of HG (74.4% vs 21.1%; p: 0.001) and IgG-HG (45.2% vs 10%; p: 0.001). Despite the frequency of HG, symptomatic IgG-HG prompting treatment with IVIG was uncommon, occurring in 10.3% of patients exposed to anti-CD20-based therapy and 1.4% of patients in the control group (p: 0.016) (Figure 1-A). The development of recurrent sino-pulmonary infections was the most frequent cause of symptomatic IgG-HG. There was a gradual downward trend in IgG levels over time in the control group as well as in Groups 1-4 prior to treatment with anti-CD20-based therapy; however, the degree of decline was significantly greater among patients in Groups 1-4 (minus 26.8 mg/dL/yr versus minus 9.3 mg/dL/yr, respectively; p: 0.001). We evaluated IgG levels subsequent to anti-CD20-based therapy. In Group 1, the IgG level rose gradually to levels close to those at time of diagnosis (increase in 10.3 mg/dL/yr). In contrast, the IgG levels fell in groups 2-4 (Group 2: minus 7.4; Group 3: minus 10.3 and Group 4: minus 0.8 mg/dL/yr). The addition of maintenance therapy following MONO or CI resulted in a long-term decline in IgG levels (Figure 1- B/C).
Conclusion: HG was frequently observed in patients with FL who received anti-CD20-based therapy, occurring in three-quarters of patients. Evaluation of the natural history of IgG levels in CD20 therapy naïve patients suggests that disease burden in FL impacts IgG levels. Additionally, anti-CD20-based therapy, except for limited treatment (Group 1) impacted IgG levels over time. Despite HG being a frequent event, few patients developed symptomatic IgG-HG. Given these data, Ig surveillance should be routinely included in follow-up of FL patients. Symptomatic HG may occur as a late event following exposure to anti-CD20-based therapy.
Younes: Novartis: Research Funding; Johnson & Johnson: Research Funding; Seattle Genetics: Honoraria; Roche: Consultancy, Honoraria, Other: Third-party medical writing assistance, under the direction of Anas Younes, was provided by Scott Malkin of Gardiner-Caldwell Communications, and was funded by F. Hoffmann-La Roche Ltd.; Curis: Research Funding; Janssen: Honoraria; Incyte: Honoraria; Merck: Honoraria; Bristol-Myers Squibb: Honoraria; Celgene: Honoraria; Bayer: Honoraria; Sanofi: Honoraria; Takeda Millenium: Honoraria. Zelenetz: Celgene: Consultancy; Amgen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal